The heart, as you know, is our main organ, but it often fails. In order to restore its normal functioning, a variety of drugs are used. One of them is Amiodarone. Analogues are much more expensive, as they are produced abroad and are not always effective.
The composition of the drug and the form of release
The drug "Amiodarone", reviews of which are very different, contains in one tablet 200 mg of the active ingredient - amiodarone hydrochloride. The drug is also produced in the form of a solution, where 3 ml accounts for 5% of the active substance.
Auxiliary components in one tablet are: crystalline microcellulose, milk sugar, maltodextrin, primellose, polyvinylpyrrolidone, magnesium stearate.
The tablets are covered with a white glaze with a light, barely perceptible creamy tint. They have a flat-cylindrical shape, as well as a chamfer and a risk. Therefore, if necessary, they can easily be divided into two equal parts.
The drug is sold in cardboard boxes, where there is one or two blisters with ten tablets. The medicine is also available in glass, polymer and plastic bottles, which have 30 or 100 tablets each.
Pharmacological mechanism of action of the drug
Amiodarone is characterized by antiarrhythmic and antianginal properties, analogues in some cases can replace it. Its antiarrhythmic effect is based on a decrease in the currents of potassium ions when exposed to cell membranes - cardiomyocytes. Causes a decrease in the sinus node, which forms bradycardia.
The use of the drug "Amiodarone" (tablets) allows you to increase the refractory segment of the conduction mechanism of the heart. It inhibits conduction along secondary pathways in patients with severe Wolff-Parkinson-White syndrome. In turn, the antianginal effect of the drug is based on a decrease in myocardial oxygen consumption and a decrease in its effect on arterial muscles. It contains iodine, which, when consumed, changes the quantitative content of thyroid hormones, as a result of which the degree of their effect on the myocardium is reduced.
"Amiodarone" has a cumulative effect, and a pronounced effect from its use occurs only after a week of regular use of the drug, and the maximum is achieved after two to three weeks.
Inside, about 40% of the drunk dose of the drug is absorbed, Cmax in plasma appears after 3-7 hours. The effect can persist for several weeks. Metabolic processes occur mainly in the liver, where the active element deethylamiodarone is formed, which is the main metabolite. Excreted with excreted bile and urine, T1 / 2 - after a single dose of the drug 3.2-20.7 hours, with prolonged therapy - after 53 ± 24 days.
The drug "Amiodarone": indications for use

The drug is prescribed for the prevention and treatment of arrhythmias, which can be life-threatening, as well as for ventricular arrhythmias, in ventricular flutter with unstable dynamics, the drug "Amiodarone" is also used.
Indications for use allow the use of the drug for:
- atrial flutter;
- supraventricular arrhythmias;
- Wolff-Parkinson-White syndrome;
- the occurrence of tachycardia;
- fibrillation state of the ventricles;
- arrhythmia resulting from heart and coronary insufficiency;
- chronic form of coronary heart disease;
- myocardial infarction.
"Amiodarone": instructions for use

The tablets are intended to be taken orally 15 minutes after a meal.
During the disease of ventricular arrhythmia, the dose recommended by the doctor is 800-1200 mg per day. It should be divided into 3-4 doses. The course of treatment in this case lasts about 5-10 days and can be extended to ensure a more stable condition of the patient, and the rate can be reduced to 600-800 mg per day. This is an intensive treatment, which later turns to prophylactic, with a lower dose of the drug content.
The therapeutic maintenance period is 7-14 days, at which time the drug is taken at 200-400 mg. Such a volume of medication is prescribed to stabilize the patient's condition in the post-rehabilitation period.
Often, the drug "Amiodarone" is prescribed when the patient is in the hospital, and the doctor can control his intake. This allows you to minimize side effects and individually adjust the dose of the drug.
In case of angina pectoris, the drug is taken twice a day, 200 mg. After two weeks, the dosage is reduced to once a day. The maximum dose of a single dose should not exceed 400 mg, and the daily dose should not exceed 1200 mg.

When prescribing such drugs to children, it should be borne in mind that the pills affect the child much faster than the adult. Therefore, the dosage is calculated based on the weight of the child. So, for one kilogram of weight should be 10 mg of the drug. This dose should be maintained for ten days of the therapeutic period or until the patient's condition improves completely. In the future, this standard is reduced to a proportion: 5 mg per kilogram of the child's live weight. The prophylactic and maintenance dose is taken on the basis of 2.4 mg of Amiodarone per kilogram of weight.
Indications for the drug are prescribed to use a solution based on the active substance amiodarone hydrochloride for acute cardiac arrhythmias. In this case, the drug is administered intravenously, injected into the body slowly with a dropper. To do this, in 250 ml of 5% for 1 or 2 hours, the drug is slowly injected at the rate of 5 mg per kilogram of live weight. At this time, the patient's condition is monitored using an ECG and measurements of blood pressure readings.
Side effects
A lot of people in our country are prescribed the medicine Amidaron. Analogs can not always replace it. But, despite the positive effect of the drug on the human body, side effects were also identified.
Negative symptoms appear mainly due to an overdose of the drug. These are disorders in the work of the cardiovascular system, such as bradycardia, malfunctions of the sinus node, heart block, and impaired coordination of movement. Also, diffuse interstitial pulmonary fibrosis, Hamman-Rich syndrome, chest pain, pulmonary fibrosis, tachypnea, bronchopulmonary spasms were noticed from the respiratory organs. Liver failure was observed when taking the drug "Amidarone". Tablets in rare cases caused jaundice, hepatitis, and sometimes cirrhosis of the liver.

Failure to comply with the dosage can cause a significant imbalance of thyroid hormones, dysthyroidism, as well as hypothyroidism and a set of extra pounds. An overdose can lead to disruption of the visual apparatus, causing the accumulation of lipids near the cornea in the pupil area. Severe skin pigmentation, photosensitivity, urticaria or erythema have also been observed. Minor rashes, alopecia, dermatitis were noticed on the face. Side effects also affected the reproductive function in men. In these cases, orchitis, impotence, and also epididymitis occurred. Rarely, thrombocytopenia, renal failure, angioedema, or vasculitis have been reported.
Contraindications for use
"Amiodarone" (reviews about it are not always positive) is selected strictly individually. There are people for whom this drug did not help or they did not notice any changes in their state of health, others began to suffocate after using it, they felt that with prolonged use it can disrupt the endocrine system, increase hormones. Therefore, before taking this drug, you must not only carefully read the instructions, but also pay attention to the indications, side effects and contraindications.
So, the medicine is not prescribed if there is an atrioventricular blockade of 2-3 degrees, with cardiogenic shock, thyrotoxicosis. The drug "Amiodarone" is not used in case of high sensitivity to its active substance and iodine. The prohibition is serious violations of cardiac conduction and attacks of bradycardia with syncope. Do not use the medicine during pregnancy and lactation. During lactation, during the period of drug treatment, breastfeeding should be stopped.
Therapeutic treatment with Amiodarone should be carefully monitored by a physician. Instructions for use of the tablet recommends using after an X-ray study of the liver, lungs and an electrocardiogram. Similar monitoring should be carried out in the future if the patient continues to take the medicine.
The severity of side effects often directly depends on the dose that the patient consumes. You should try to use the drug as little as possible and in minimal quantities. Often causes a heart rhythm failure to cancel the drug "Amiodarone".
Instructions for use - reviews say that, despite the side effects, this drug has saved more than one life - claims that the pharmacological effect of the drug persists for another two weeks after its cancellation.

During therapeutic treatment, it is necessary to avoid prolonged exposure to the sun, do not take open sunbathing. The drug should be administered with extreme caution to the elderly, under general anesthesia or during the course of oxygen treatment. It should be used with caution by drivers of vehicles and persons whose profession is associated with a special concentration of attention.
In cases of overdose, there may be an aggravation of side effects, as well as hypotension, arrhythmia. Bradycardia or AV conduction failure may occur. Excess can lead to liver dysfunction.
In this case, immediate gastric lavage is recommended, activated charcoal is prescribed, saline laxative solutions are recommended. With bradycardia, injections of atropine are made, pacing is used.
Interaction with other drugs
With caution, the drug should be used simultaneously with other drugs, especially if they are from the same antiarrhythmic group. You should not combine his reception with Erythromycin, Pentamidine and Vincamine. There is a risk of developing polymorphic ventricular tachycardia in combination with Sultopride. It is not recommended to combine the intake of CCBs and beta-blockers with Amphotericin B and drugs that cause a laxative effect, diuretics. Use the drug with caution in combination with corticosteroids, antidepressants, Astemizol, Terfenadine. It is able to significantly enhance the effect of such drugs as: "Warfarin", "Phenytoin", "Cyclosporine" or "Digoxin". When used simultaneously with Cimetidine, it slows down the metabolic process in the body.
Expiration date and storage standards
The drug "Amiodarone", whose action is aimed at eliminating tachycardia, ventricular fibrillation, tachyarrhythmia and reducing atrial flutter, belongs to the category of drugs of group "B". The shelf life of the drug does not exceed three years. Tablets should be stored in a dark, cool place out of the reach of children.
It is necessary to strictly control the use of the drug "Amiodarone". A prescription for its use is written only by a doctor. In this case, self-medication should be completely excluded.
Drug analogues

If suddenly the drug "Amiodarone" does not fit, analogues can be found in any pharmacy. Among them, one can single out such drugs as: "Amiodarone Belupo" and "Amiodarone Aldaron", "Angoron" and "Atlansil", "Kordaron" or "Kordinil" can also replace this medicine. Similar to "Amiodarone" in their effect on the human body "Medakoron" and "Palpitin". In some cases, the drug is replaced by Sedacoron and Sandoz.
These drugs either contain the same active ingredient, or are similar in their antiarrhythmic action to the drug Amiodarone. Analogues are often produced abroad and cost several times more.
Amiodarone is an antiarrhythmic drug.
Release form and composition
Amiodarone tablets are prepared containing 200 mg of amiodarone hydrochloride.
Auxiliary components of the drug are: lactose monohydrate, magnesium stearate, colloidal silicon dioxide, microcrystalline cellulose, sodium carboxymethyl starch, corn starch, povidone.
In blisters of 10 pieces.
Indications for the use of Amiodarone
According to the instructions, Amiodarone is indicated for the prevention of paroxysmal arrhythmias, namely:
- Ventricular arrhythmias that threaten the patient's life (ventricular fibrillation, ventricular tachycardia);
- Supraventricular arrhythmias (including those with organic heart disease or when it is impossible to use alternative antiarrhythmic therapy);
- Atrial fibrillation (atrial fibrillation), atrial flutter;
- Attacks of recurrent sustained supraventricular paroxysmal tachycardia in patients with Wolff-Parkinson-White syndrome.
Contraindications
According to the instructions Amiodarone is contraindicated in:
- Severe arterial hypotension;
- Sinus node weakness syndrome (sinoatrial blockade, sinus bradycardia, lack of a pacemaker);
- Atrioventricular blockade of 2-3 degrees, two- and three-beam blockade (in the absence of a pacemaker);
- The period of pregnancy and breastfeeding;
- Impaired thyroid function (hyper- or hypothyroidism);
- Hypomagnesemia, hypokalemia;
- Interstitial lung diseases;
- Hypersensitivity to amiodarone, iodine or auxiliary components of the drug;
- Congenital or acquired prolongation of the QT interval;
- Simultaneous use of monoamine oxidase inhibitors;
- Lactose intolerance, lack of lactase or glucose-galactose malabsorption;
- Under the age of 18 (the safety and efficacy of amiodarone have not been established);
- Simultaneous administration with drugs that prolong the QT interval and cause the development of paroxysmal tachycardia.
In the use of Amiodarone, care should be taken when:
- Bronchial asthma;
- liver failure;
- Chronic heart failure;
- Elderly (increases the likelihood of developing severe bradycardia);
- AV blockade 1 degree.
Method of application and dosage of Amiodarone
According to the instructions, Amiodarone is intended for internal use. Tablets are taken before meals with plenty of water. The dosage of the drug is set by the attending physician individually.
The loading dose of Amiodarone is 60-800 mg per day (not more than 1200 mg) for 5-8 days. Upon reaching the desired effect, the dosage of the drug is reduced to 100-400 mg per day, divided into 2 doses.
Since amiodarone has a long half-life, it can be taken every other day or intermittently twice a week.
Side effects of amiodarone
The use of Amiodarone can cause the following side effects:
- Cardiovascular system: moderate bradycardia, sinoatrial blockade, proarrhythmic effect, AV blockade of varying degrees, sinus arrest. With prolonged use of the drug, progression of symptoms of chronic heart failure is possible;
- Digestive system: nausea, vomiting, taste disturbance, loss of appetite, increased activity of liver enzymes, heaviness in the epigastrium, acute toxic hepatitis, jaundice, liver failure;
- Respiratory system: interstitial or alveolar pneumonitis, pulmonary fibrosis, pleurisy, obliterating bronchitis with pneumonia, including fatal, acute respiratory syndrome, pulmonary hemorrhage, bronchospasm (especially in patients with bronchial asthma);
- Sense organs: optic neuritis, deposition of lipofuscin in the corneal epithelium;
- Endocrine system: an increase in the level of the hormone T4, accompanied by a slight decrease in T3 (does not require discontinuation of treatment with Amiodarone if the thyroid function is not impaired). With prolonged use, hypothyroidism may develop, less often - hyperthyroidism, requiring discontinuation of the drug. Very rarely, a syndrome of impaired secretion of ADH may occur;
- Nervous system: extrapyramidal disorders, tremor, nightmares, sleep disturbances, peripheral neuropathy, myopathy, cerebellar ataxia, headache, brain pseudotumor;
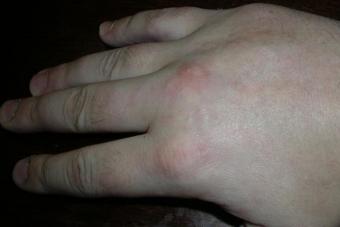
- Skin reactions: photosensitivity, with prolonged use of the drug - lead-blue or blue pigmentation of the skin, erythema, exfoliative dermatitis, skin rash, alopecia, vasculitis;
- Laboratory indicators: aplastic or hemolytic anemia, thrombocytopenia;
- Other adverse reactions: reduced potency, epididymitis.
special instructions
Before starting therapy with Amiodarone, as well as every three months during treatment, ECG monitoring, X-ray examination of the lungs and liver function should be performed. Also, before starting therapy, it is recommended to check the content of electrolytes in the blood plasma.
The frequency and severity of adverse reactions of Amiodarone directly depend on the dosage of the drug, so it should be used in the minimum allowable doses.
Cancellation of Amiodarone may cause recurrence of cardiac arrhythmias.
As a rule, the pharmacological effect of Amiodarone persists for another two weeks after its withdrawal.
The drug contains iodine, which can interfere with the results of tests for the accumulation of radioactive iodine in the thyroid gland. Before starting treatment and during drug therapy, you should regularly donate blood to the level of thyroid hormones.
Amiodarone analogs
Amiodarone analogues are the following drugs:
- Angoron;
- Aldaron;
- Atlansil;
- Kordaron;
- Cordinyl;
- Medacorone;
- Palpitin;
- Sedacoron.
Terms and conditions of storage
Amiodarone should be stored in a dry, dark place at a cool temperature. The shelf life of the drug is 2 years from the date of manufacture.
Found a mistake in the text? Select it and press Ctrl + Enter.
One tablet of Amiodarone contains 200 mg amiodarone hydrochloride and excipients such as: lactose, corn starch, alginic acid, low molecular weight povidone and magnesium stearate.
Release form
Amiodarone is available in tablets in blister packs of 10 pieces or in a glass jar of 30 pieces. The drug is packaged in cardboard packs that can hold 30 or 60 tablets.
Solution for intravenous administration (prescription in Latin): Rp.: Sol. 300 mg Amiodaroni diluitur Dextrosum 5% - 20 ml.
pharmachologic effect
It has antiarrhythmic, coronary vasodilating and antianginal effects.
Pharmacodynamics and pharmacokinetics
Amiodarone - an active substance that can facilitate the work of the heart without significantly changing cardiac output and contractility of the heart muscle myocardium . At the same time, the drug increases coronary blood flow by reducing resistance in the arteries of the heart, and also reduces heart rate and blood pressure due to peripheral vasodilating effect . This significantly reduces the level of oxygen consumption by the myocardium and at the same time increases the energy reserves of the myocardium by increasing the content creatine phosphate and glycogen .
Indications for the use of Amiodarone
Used for treatment and prevention paroxysmal arrhythmias :
- ventricular that are life threatening, as well as in patients with Chagas myocarditis ;
- ventricular ;
- prevention ventricular fibrillation , among other things - after the events cardioversion ;
- flicker paroxysm ;
- atrial flutter ;
- atrial extrasystole or ventricular ;
- arrhythmias appearing on the background chronic cardiac or coronary insufficiency ;
- parasystole ;
Indications for the use of amiodarone are also supraventricular arrhythmias in cases of ineffectiveness or inability to use other therapy, which is usually associated with WPW syndrome.
Contraindications
- sinus bradycardia ;
- weak sinus syndrome ;
- sinoatrial or 2nd and 3rd degree (without the use pacemaker );
- cardiogenic shock ;
- collapse ;
- hypokalemia ;
- arterial hypotension ;
- (insufficient secretion of thyroid hormones);
- interstitial lung disease ;
- reception MAO inhibitors ;
- period and ;
- hypersensitivity to the components of Amiodarone or to;
- Caution should be used in children and adolescents under 18 years of age.
Side effects
The use of Amiodarone tablets can cause the following side effects with respect to certain organs and systems:
- The cardiovascular system: sinus bradycardia (refractory to m-anticholinergics ), AV block , vasculitis , with long-term use - progression of CHF , ventricular arrhythmia type " pirouette “, strengthening the existing arrhythmias or its occurrence, with parenteral use - decrease in blood pressure .
- Endocrine system: developing hypo - or hyperthyroidism .
- Respiratory system: long-term use may cause cough , interstitial pneumonia or and also pulmonary fibrosis , pleurisy. When used parenterally, it is possible bronchospasm especially in people with severe respiratory failure.
- Digestive system: most common nausea , vomit , or , severity in epigastrium , decreases, taste sensations are dulled, less often - increased activity liver transaminases , in case of long-term use - toxic hepatitis , cholestasis , icteric discoloration of the skin , as well as .
- Central and peripheral nervous system: possible , asthenia , auditory . In case of prolonged use - peripheral neuropathy , extrapyramidal manifestations, impaired memory, sleep, ataxia , neuritis optic nerve . When administered parenterally, it may develop intracranial hypertension .
- sense organs: uveitis (inflammation of the choroid of the eye of various localization), deposition glycolipoprotein — lipofuscin v cornea , which can manifest itself in bright light in the form of violations: complaints of luminous dots or the so-called "veil before the eyes", in addition, it is possible microretinal detachment .
- Hematopoietic organs: thrombocytopenia , hemolytic or aplastic anemia .
- Skin: rash , defeat in the form exfoliative , photosensitivity Rarely, there were manifestations in the form of a gray-blue staining of the skin.
- Others: epididymitis and decline, myopathy , with parenteral use, it is possible and elevated sweating .
The use of the drug in elderly patients significantly increases the risk of developing severe forms bradycardia .
Amiodarone tablets, instructions for use (Method and dosage)
Amiodarone tablets should be taken orally, before meals, with the necessary amount of water to swallow. Instructions for use Amiodarone suggests an individual dosing regimen, which must be established and adjusted by the attending physician.
Standard dosing regimen:
- The loading (otherwise saturating) initial dose for inpatient treatment, which is divided into several doses, is 600–800 mg per day, with the maximum allowable daily dose being up to 1200 mg. It should be borne in mind that the total dose should be 10 g, usually it is achieved in 5-8 days.
- For outpatient treatment, an initial dose is prescribed in the range of 600–800 mg per day, which is divided into several doses, also reaching a total dose of not more than 10 g, however, in 10–14 days.
- To continue the course of treatment with Amiodarone, it is enough to take 100-400 mg per day. Attention! The lowest effective maintenance dose is used.
- To avoid cumulation of the drug, it is necessary to take tablets either every other day or with a break of 2 days, 1 time per week.
- The average single dose with a therapeutic effect is 200 mg.
- The average daily dose is 400 mg.
- The maximum allowable dose is not more than 400 mg at a time, not more than 1200 mg for 1 knock.
- For children, the dose is usually in the range of 2.5-10 mg per day.
Overdose
A single significant dose can cause:
- decline;
- bradycardia or ;
- violation of the normal functioning of the liver;
- atrioventricular block .
As a treatment prescribed gastric lavage , symptomatic measures, with the development bradycardia — , β1-agonists , in extreme cases - pacing .
specific does not exist, turns out to be ineffective.
Interaction
When this drug is used simultaneously with the following drugs, various reactions may occur:
- Class 1A antiarrhythmics and Disopyramide , Procainamide , Quinidine increase the cardiac interval QT and increase the risk of developing ventricular tachycardia "pirouette".
- Laxatives that cause hypokalemia , as well as diuretics , corticosteroids, including in / in , Tetracosactide , in combination with amiodarone increase the risk of developing ventricular arrhythmias.
- General anesthesia , oxygen therapy - the risk of developing bradycardia, cardiac conduction disorders, arterial hypotension, a decrease in the stroke V heart.
- tricyclic antidepressants, phenothiazines , Astemizol and also cause prolongation of the QT interval and the risk of developing ventricular arrhythmias (most often of the "pirouette" type).
- , Phenprocoumon , Acenocoumarol enhance the anticoagulant effect and increase the risk of bleeding.
- Vincamine , Sultopride ,
Amiodarone is an antiarrhythmic drug. It is prescribed for patients with coronary heart disease with angina syndromes of rest and exertion.
Antiarrhythmic action is characterized by the impact on the electrophysiological process in the myocardium. The drug is able to lengthen the action potential of cardiomyocytes, increase the effective refractory period of the ventricles and atria. The antianginal effect is explained by the coronary dilating effect, a decrease in the oxygen demand of the heart muscle.
On this page you will find all the information about Amiodarone: complete instructions for use for this drug, average prices in pharmacies, complete and incomplete analogues of the drug, as well as reviews of people who have already used Amiodarone. Want to leave your opinion? Please write in the comments.
Clinical and pharmacological group
Antiarrhythmic agent of class 3, has antianginal action.
Terms of dispensing from pharmacies
Released by prescription.
Prices
How much does amiodarone cost? The average price in pharmacies is at the level of 80 rubles.
Release form and composition
Produced in the form of white tablets of a round, flat-cylindrical shape, having a one-sided chamfer and a risk.
- Amiodarone hydrochloride - in 1 tab. 200 mg.
- Contains the following excipients: povidone, corn starch, Mg stearate, colloidal silicon dioxide, Na starch glycolate, microcrystalline cellulose.
Tablets are packaged in blisters (10 pcs), cardboard packaging.
Pharmacological effect
Amiodarone is a class III antiarrhythmic drug. It also has alpha- and beta-adrenergic blocking, antianginal, antihypertensive and coronary dilating effects.
The drug blocks inactivated potassium channels in the cell membranes of cardiomyocytes. To a lesser extent, it affects sodium and calcium channels. By blocking inactivated "fast" sodium channels, it produces effects that are characteristic of class I antiarrhythmic drugs. Amiodarone causes bradycardia by inhibiting the slow depolarization of the sinus node cell membrane, and also inhibits atrioventricular conduction (the effect of class IV antiarrhythmic drugs).
The antiarrhythmic effect of the drug is due to its ability to increase the duration of the action potential of cardiomyocytes and the refractory (effective) period of the ventricles and atria of the heart, the bundle of His, the AV node and Purkinje fibers, resulting in a decrease in the automatism of the sinus node, excitability of cardiomyocytes and slows down AV conduction.
The antianginal effect of the drug is due to a decrease in the resistance of the coronary arteries and a decrease in myocardial oxygen demand due to a decrease in heart rate, which ultimately leads to an increase in coronary blood flow. The drug does not significantly affect systemic blood pressure.
Indications for use
According to the instructions, Amiodarone is indicated for the prevention of paroxysmal arrhythmias, namely:
- (atrial fibrillation), atrial flutter;
- Ventricular arrhythmias that threaten the patient's life (ventricular fibrillation, ventricular tachycardia);
- Supraventricular arrhythmias (including those with organic heart disease or when it is impossible to use alternative antiarrhythmic therapy);
- Attacks of recurrent sustained supraventricular paroxysmal tachycardia in patients with Wolff-Parkinson-White syndrome.
Contraindications
This drug is contraindicated in SA and AV blockade of 2-3 degrees, sinus bradycardia, collapse, hypersensitivity, cardiogenic shock, hypokalemia, pulmonary interstitial diseases, hypothyroidism, thyrotoxicosis, during pregnancy, lactation and taking MAO inhibitors.
In addition, according to the instructions, Amiodarone is prescribed with caution to people suffering from renal failure, chronic heart failure and bronchial asthma. Also, this remedy must be carefully taken by children under 18 years of age and elderly patients.
Instructions for use Amiodarone
The instructions for use indicate that Amiodarone tablets should be taken orally, before meals, with the necessary amount of water to swallow. Instructions for use Amiodarone suggests an individual dosing regimen, which must be established and adjusted by the attending physician.
Standard dosing regimen:
- The loading (otherwise saturating) initial dose for inpatient treatment, which is divided into several doses, is 600–800 mg per day, with the maximum allowable daily dose being up to 1200 mg. It should be borne in mind that the total dose should be 10 g, usually it is achieved in 5-8 days.
- For outpatient treatment, an initial dose is prescribed in the range of 600–800 mg per day, which is divided into several doses, also reaching a total dose of not more than 10 g, however, in 10–14 days.
- To continue the course of treatment with Amiodarone, it is enough to take 100-400 mg per day. Attention! The lowest effective maintenance dose is used.
- To avoid cumulation of the drug, it is necessary to take tablets either every other day or with a break of 2 days, 1 time per week.
- The average single dose with a therapeutic effect is 200 mg.
- The average daily dose is 400 mg.
- The maximum allowable dose is not more than 400 mg at a time, not more than 1200 mg for 1 knock.
- For children, the dose is usually in the range of 2.5-10 mg per day.
Side effects
The use of Amiodarone can cause the following side effects:
- Nervous system: extrapyramidal disorders, tremor, nightmares, sleep disturbances, peripheral neuropathy, myopathy, cerebellar ataxia, headache, brain pseudotumor;
- Skin reactions: photosensitivity, with prolonged use of the drug - lead-blue or blue pigmentation of the skin, erythema, exfoliative dermatitis, skin rash, alopecia, vasculitis;
- Respiratory system: interstitial or alveolar pneumonitis, pulmonary fibrosis, pleurisy, obliterating bronchitis with pneumonia, including fatal, acute respiratory syndrome, pulmonary hemorrhage, bronchospasm (especially in patients with bronchial asthma);
- Sense organs: optic neuritis, deposition of lipofuscin in the corneal epithelium;
- Endocrine system: an increase in the level of the hormone T4, accompanied by a slight decrease in T3 (does not require discontinuation of treatment with Amiodarone if the thyroid function is not impaired). With prolonged use, hypothyroidism may develop, less often - hyperthyroidism, requiring discontinuation of the drug. Very rarely, a syndrome of impaired secretion of ADH may occur;
- Cardiovascular system: moderate bradycardia, sinoatrial blockade, proarrhythmic effect, AV blockade of varying degrees, sinus arrest. With prolonged use of the drug, progression of symptoms of chronic heart failure is possible;
- Digestive system: nausea, vomiting, taste disturbance, loss of appetite, increased activity of liver enzymes, heaviness in the epigastrium, acute toxic hepatitis, jaundice, liver failure;
- Laboratory indicators: aplastic or hemolytic anemia, thrombocytopenia;
- Other adverse reactions: reduced potency, epididymitis.
Overdose
Taking large doses of amiodarone can lead to the following conditions:
- Hypotension;
- bradycardia;
- AV block;
- asystole;
- Cardiogenic shock;
- liver dysfunction;
- Heart failure.
The patient should be immediately taken to a medical facility. Treatment of an overdose of amiodarone is aimed at detoxifying the body (gastric lavage, taking enterosorbents) and eliminating symptoms.
special instructions
Taking the drug is possible only after the appointment of a doctor who determines the treatment regimen and dosage based on clinical trial data and ECG. The following special instructions must also be taken into account:
- Before starting the use of the drug, it is recommended to conduct a study of the functional activity of the thyroid gland and the level of its hormones in the blood.
- With prolonged use, ECG monitoring of the heart, determination of the level of thyroid hormones and liver enzymes in the blood is mandatory.
- With increased caution and with constant ECG monitoring of the heart, Amiodarone tablets are prescribed when used together with beta-blockers, laxatives and diuretics that remove potassium ions from the body (potassium-sparing diuretics - furosemide), anticoagulants (warfarin), some antibiotics (rifampicin) and antiviral agents (especially drugs that inhibit the reverse transcriptase of viruses).
- It is impossible to combine the use of Amiodarone tablets with other antiarrhythmic drugs, as this will lead to an increase in its effects and the development of disturbances in the functional activity of the heart. The combination with antimalarial drugs, antibiotics of the macrolide group, fluoroquinolones is also excluded.
- In case of cough and shortness of breath, an X-ray examination of the chest organs is performed to differentiate the inflammatory pathology of the respiratory organs.
- While taking Amiodarone tablets, it is necessary to abandon activities associated with an increased concentration of attention and requiring a high speed of psychomotor reactions.
In pharmacies, the drug is dispensed only by prescription.
drug interaction
- Fluoroquinolones;
- Beta-blockers;
- laxatives;
- Antiarrhythmic drugs of the 1st class;
- Antipsychotics;
- tricyclic antidepressants;
- macrolides;
- Antimalarial.
The joint appointment of these drugs with amiodarone can lead to severe side effects, often life-threatening.
The pharmacokinetics of the drug is affected by:
- Cholinesterase inhibitors;
- Orlistat;
- Colestyramine;
- Anticoagulants;
- cardiac glycosides;
- antiviral drugs;
- Cimetidine.
Amiodarone itself is able to influence the concentrations of cyclosporine, lidocaine, statins, sodium iodide.
INN: Amiodarone
Manufacturer: Open Joint Stock Company "Borisov Plant of Medical Preparations" (JSC "BZMP")
Anatomical-therapeutic-chemical classification: Amiodarone
Registration number in the Republic of Kazakhstan: No. RK-LS-5 No. 016246
Registration period: 12.11.2015 - 12.11.2020
Instruction
Tradename
Amiodarone
International non-proprietary name
Amiodarone
Dosage form
Tablets 200 mg
Compound
One tablet contains:
active substance- amiodarone hydrochloride (in terms of 100% substance) 200 mg,
Excipients: lactose monohydrate, potato starch, povidone, calcium stearate.
Description
Tablets of white or almost white color, ploskotsilindrichesky, with risk and a facet.
Farmacotherapeutic group
Drugs for the treatment of heart disease. Class III antiarrhythmic drugs. Amiodarone.
ATX code C01BD01.
Pharmacological properties
Pharmacokinetics
Absorption is slow and variable - 30-50%, bioavailability - 30-50%. The maximum concentration in blood plasma is observed after 3-7 hours. The range of therapeutic plasma concentration is 1-2.5 mg / l (but when determining the dose, the clinical picture must also be taken into account). The volume of distribution is 60 l, which indicates an intensive distribution in the tissue. It has high fat solubility, is found in high concentrations in adipose tissue and organs with good blood supply (concentration in adipose tissue, liver, kidneys, myocardium is higher than in plasma, respectively, 300, 200, 50 and 34 times). Features of the pharmacokinetics of amiodarone necessitate the use of the drug in high loading doses. Penetrates through the blood-brain barrier and the placenta (10-50%), secreted in breast milk (25% of the dose received by the mother). Communication with plasma proteins - 95% (62% - with albumin, 33.5% - with beta-lipoproteins).
Metabolized in the liver. The main metabolite, deethylamiodarone, is pharmacologically active and may enhance the antiarrhythmic effect of the main compound. Possibly also metabolized by deiodination (at a dose of 300 mg, approximately 9 mg of elemental iodine is released). With prolonged treatment, iodine concentrations can reach 60-80% of the concentration of amiodarone. It is an inhibitor of the CYP2C9, CYP2D6 and CYP3A4, CYP3A5, CYP3A7 enzyme systems in the liver.
Given the ability to accumulate and the associated large variability in pharmacokinetic parameters, data on the elimination half-life are contradictory. Removal of amiodarone after oral administration is carried out in 2 phases: the initial period is 4-21 hours, in the second phase, the half-life is 25-110 days. After long-term oral administration, the mean half-life is 40 days (this is important when choosing a dose, because it may take at least 1 month to stabilize the new plasma concentration, while complete elimination can last 61 days (more 4 months).
Excreted with bile (85-95%), less than 1% of the oral dose is excreted by the kidneys (therefore, with impaired renal function, there is no need to change the dosage). Amiodarone and its metabolites are not subject to dialysis.
Pharmacodynamics
Class III antiarrhythmic drug (repolarization inhibitor). It also has antianginal, coronary-dilating, alpha- and beta-blocking and hypotensive effects.
The antianginal effect is due to coronary dilating and antiadrenergic action, a decrease in myocardial oxygen demand.
It has an inhibitory effect on the alpha and beta adrenoreceptors of the cardiovascular system (without their complete blockade). Reduces sensitivity to hyperstimulation of the sympathetic nervous system, the tone of the coronary vessels; increases coronary blood flow; slows down the heart rate; increases the energy reserves of the myocardium (by increasing the content of creatine sulfate, adenosine and glycogen).
Antiarrhythmic action is due to the influence on electrophysiological processes in the myocardium; lengthens the action potential of cardiomyocytes, increasing the effective refractory period of the atria, ventricles, atrioventricular node, bundle of His and Purkinje fibers, additional pathways for conducting excitation.
By blocking inactivated "fast" sodium channels, it has effects characteristic of class I antiarrhythmic drugs.
It inhibits the slow (diastolic) depolarization of the sinus node cell membrane, causing bradycardia, inhibits atrioventricular conduction (the effect of class IV antiarrhythmics).
It is similar in structure to thyroid hormones. The content of iodine is about 37% of its mol. masses. It affects the metabolism of thyroid hormones, inhibits the conversion of T4 to T3 (thyroxine-5-deiodinase blockade) and blocks the capture of these hormones by cardiocytes and hepatocytes, which leads to a weakening of the stimulating effect of thyroid hormones on the myocardium.
The onset of action (even when using "loading" doses) is from 2-3 days to 2-3 months, the duration of action varies from several weeks to months (determined in plasma for 9 months after stopping its administration).
Indications for use
Amiodarone therapy can only be carried out in hospitals or on an outpatient basis under the supervision of a cardiologist.
For the treatment of severe arrhythmias that do not respond to other drugs, or when other drugs cannot be prescribed.
Tachyarrhythmias associated with Wolff-Parkinson-White syndrome.
Atrial fibrillation and flutter, in case other drugs cannot be prescribed.
Tachyarrhythmias of a paroxysmal nature, including atrial, atrioventricular and ventricular tachycardia, ventricular fibrillation, when other drugs cannot be prescribed.
Dosage and administration
Initial treatment
The usual dosing regimen is 600 mg / day - 3 tablets per day, divided into 2-3 doses, for 8-10 days.
In some cases, higher doses (4 or 5 tablets per day) may be used at the beginning of treatment, but only for a short time and under electrocardiographic control.
Supportive care
The minimum effective dose should be determined, in accordance with the individual response, it can range from ½ tablet per day (1 tablet every other day) to 2 tablets per day.
The average single therapeutic dose is 200 mg, the average therapeutic daily dose is 400 mg, the maximum single dose is 400 mg, the maximum daily dose is 1200 mg.
Side effects
Frequency: very often (10% or more), often (1% or more; less than 10%), infrequently (0.1% or more; less than 1%), rarely (0.01% or more; less than 0.1 %), very rarely (less than 0.01%, including individual cases), the frequency is unknown (the frequency cannot be determined from the available data).
Very often (10% or more)
Nausea, vomiting, loss of appetite, dullness or loss of taste, a feeling of heaviness in the epigastrium, an isolated increase in the activity of "liver" transaminases (1.5 - 3 times higher than normal)
Micro-deposits in the cornea, almost always present in adults, are usually localized in the area under the pupil and are not a contraindication to continued treatment. In exceptional cases, they may be accompanied by the perception of colored and blinding light or blurred vision. Micro-deposits in the cornea, which are formed by a complex of lipids, always disappear after the treatment is stopped.
In the absence of any clinical symptoms of dysthyroidism, the level of "dissociated" thyroid hormone (increased T4 levels with normal or slightly reduced T3 levels) is not a reason to interrupt treatment.
Often (1% or more; less than 10%)
Moderate bradycardia (dose-dependent);
Acute toxic hepatitis with an increase in the activity of "liver" transaminases and / or jaundice, including the development of liver failure, incl. fatal;
Interstitial or alveolar pneumonitis, bronchiolitis obliterans with pneumonia, incl. fatal, pleurisy, pulmonary fibrosis;
With prolonged use, hypothyroidism, hyperthyroidism may develop (possibly fatal, drug withdrawal is required);
Grayish or bluish pigmentation of the skin (with prolonged use; disappears after stopping the drug);
Tremor and other extrapyramidal symptoms, sleep disorders, incl. "nightmare" dreams
Uncommon (0.1% or more; less than 1%)
SA and AV blockade of various degrees, proarrhythmic effect (the emergence of new or aggravation of existing arrhythmias, including cardiac arrest);
Conduction disorders (sinoauricular blockade of varying degrees)
Rarely:
Peripheral neuropathy (sensory, motor, mixed) and/or myopathy
Very rare (less than 0.01%, including isolated cases)
Severe bradycardia, sinus arrest (in patients with sinus node dysfunction and elderly patients);
Chronic liver failure (pseudo-alcoholic hepatitis, cirrhosis), incl. fatal;
Bronchospasm in patients with severe respiratory failure (especially in patients with bronchial asthma), acute respiratory syndrome, incl. fatal;
Optic neuritis/optic neuropathy.
Syndrome of inappropriate ADH secretion CHCAD/RSIADH (hyponatremia)
Erythema (with simultaneous radiation therapy), skin rash, exfoliative dermatitis (the relationship with the drug has not been established), alopecia.
Cerebellar ataxia, benign intracranial hypertension (pseudotumor of the brain), headache, vertigo;
Vasculitis;
epididymitis;
Violation of potency (the relationship with the drug has not been established);
With prolonged use of thrombocytopenia, hemolytic and aplastic anemia;
Renal failure with a moderate increase in creatinine;
Frequency unknown (frequency cannot be determined from available data)
Pulmonary bleeding;
Cases of bone marrow granuloma;
Cases of angioedema.
Contraindications
Hypersensitivity (including to iodine);
Sick sinus syndrome;
sinus bradycardia;
Sinoatrial blockade;
Atrioventricular block II-III Art. (without the use of a pacemaker);
Cardiogenic shock;
hypokalemia;
Collapse;
arterial hypotension;
Hypothyroidism;
thyrotoxicosis;
Interstitial lung disease;
Children and adolescents up to 18 years of age;
Taking monoamine oxidase inhibitors.
Two- and three-beam blockade (without the use of a pacemaker);
Hypomagnesemia;
Hypothyroidism;
Hyperthyroidism;
Congenital or acquired prolongation of the QT interval;
Simultaneous administration of drugs that prolong the QT interval and cause paroxysmal tachycardia (including polymorphic ventricular pirouette type);
Pregnancy and lactation.
Carefully: chronic insufficiency III and IV degree, AV blockade stage I, liver failure, bronchial asthma, old age (high risk of developing severe bradycardia)
Drug Interactions
Contraindicated combinations (risk of developing polymorphic ventricular tachycardia of the "pirouette" type): class 1a antiarrhythmic drugs (quinidine, hydroquinidine, disopyramide, procainamide), class III (dofetilide, ibutilide, bretylium tosylate), sotalol; bepridil, vincamine, phenothiazines (chlorpromazine, cyamemazine, levomepromazine, thioridazine, trifluoperazine, fluphenazine), benzamides (amisulpride, sultopride, sulpiride, tiapride, veraliprid), butyrophenones (droperidol, haloperidol), sertindole, pimozide; tricyclic antidepressants, cisapride, macrolides (IV erythromycin, spiramycin), azoles, antimalarial drugs (quinine, chloroquine, mefloquine, halofantrine, lumefantrine); pentamidine (parenteral), difemanil methyl sulfate, mizolastine, astemizole, terfenadine, fluoroquinolones (including moxifloxacin).
Not recommended combinations: beta-blockers, slow calcium channel blockers (verapamil, diltiazem) - the risk of impaired automatism (severe bradycardia) and conduction; laxative drugs that stimulate intestinal motility - the risk of developing ventricular tachycardia of the "pirouette" type against the background of hypokalemia caused by laxative drugs, cardiac glycosides - impaired automatism (severe bradycardia) and AV conduction (increased digoxin concentration);
Combinations requiring caution:
Diuretics that cause hypokalemia, amphotericin B (iv), systemic glucocorticosteroids, tetracosactide - the risk of developing ventricular arrhythmias, incl. ventricular tachycardia of the "pirouette" type;
Procainamide - the risk of side effects of procainamide (amiodarone increases the plasma concentration of procainamide and its metabolite N-acetylprocainamide);
Indirect anticoagulants (warfarin) - amiodarone increases the concentration of warfarin (the risk of bleeding) by inhibiting the CYP2C9 isoenzyme;
Esmolol - violation of contractility, automatism and conductivity (suppression of compensatory reactions of the sympathetic nervous system);
Phenytoin, fosphenytoin - the risk of developing neurological disorders (amiodarone increases the concentration of phenytoin due to inhibition of the CYP2C9 isoenzyme);
Flecainide - amiodarone increases its concentration (due to inhibition of the CYP2D6 isoenzyme);
Drugs metabolized with the participation of the CYP3A4 isoenzyme (cyclosporine, fentanyl, lidocaine, tacrolimus, sildenafil, midazolam, triazolam, dihydroergotamine, ergotamine, statins, including simvastatin - amiodarone increases their concentration (the risk of developing their toxicity and / or increasing pharmacodynamic effects);
Orlistat reduces the concentration of amiodarone and its active metabolite; clonidine, guanfacine, cholinesterase inhibitors (donepezil, galantamine, rivastigmine, tacrine, ambenonium chloride, pyridostigmine, neostigmine), pilocarpine - the risk of developing severe bradycardia;
Cimetidine, grapefruit juice slow down the metabolism of amiodarone and increase its plasma concentration;
Medicines for inhalation anesthesia - the risk of developing bradycardia (resistant to the administration of atropine), lowering blood pressure, conduction disturbances, reduced cardiac output, acute respiratory distress syndrome, incl. fatal, the development of which is associated with high oxygen concentrations;
Radioactive iodine - amiodarone (contains iodine in its composition) can interfere with the absorption of radioactive iodine, which can distort the results of a radioisotope study of the thyroid gland;
Rifampicin and preparations of St. John's wort (potent inducers of the CYP3A4 isoenzyme) reduce the concentration of amiodarone in plasma; HIV protease inhibitors (CYP3A4 isoenzyme inhibitors) may increase plasma concentrations of amiodarone;
Clopidogrel - a decrease in its plasma concentration is possible;
Dextromethorphan (a substrate of CYP3A4 and CYP2D6 isoenzymes) - an increase in its concentration is possible (amiodarone inhibits the CYP2D6 isoenzyme).
special instructions
Chronic heart failure (FC III-IV according to the NYHA classification), AV blockade I stage, liver failure, bronchial asthma, old age (high risk of developing severe bradycardia).
Before starting therapy, it is necessary to conduct an ECG, X-ray examination of the lungs, evaluate the function of the thyroid gland (hormone concentration), liver (transaminase activity) and the concentration of electrolytes (potassium) in the plasma.
During the period of treatment, transaminases are periodically analyzed (with an increase of 3 times or doubling in the case of initially increased activity, the dose is reduced, up to the complete cessation of therapy) and ECG (the width of the QRS complex and the duration of the QT interval). An increase in the QTc interval of no more than 450 ms or no more than 25% of the original value is acceptable. These changes are not a manifestation of the toxic effect of the drug, but require monitoring for dose adjustment and evaluation of the possible proarrhythmic effect of amiodarone.
An annual X-ray examination of the lungs, an examination of the function of external respiration 1 every six months, a thyroid-stimulating hormone test before starting treatment and then on a regular basis during treatment and a few months after stopping treatment are recommended. In the absence of clinical signs of thyroid dysfunction, treatment should not be discontinued. The appearance of shortness of breath or unproductive cough may be associated with the toxic effect of amiodarone on the lungs. Respiratory disorders are mostly reversible with early withdrawal of amiodarone. Early withdrawal of amiodarone associated with glucocorticosteroid therapy or not associated with it leads to regression of disorders. Clinical symptoms usually disappear within 3-4 weeks, and then there is a slower recovery of the x-ray picture and lung function (several months).
To prevent the development of photosensitivity, it is recommended to avoid sun exposure or use special sunscreens.
If blurred vision or reduced visual acuity occurs while taking amiodarone, it is recommended to conduct a complete ophthalmological examination, including fundoscopy. Cases of neuropathy and / or optic neuritis require a decision on the advisability of using amiodarone.
When canceled, relapses of rhythm disturbances are possible.
Due to the presence of lactose in the preparation, it is not recommended to take it in patients with congenital galactose intolerance, Lapp lactase deficiency, glucose-galactose malabsorption.
After cancellation, the pharmacodynamic effect persists for 10-30 days.
Contains iodine (in 200 mg - 75 mg of iodine), so it may affect the results of tests for the accumulation of radioactive iodine in the thyroid gland.
When performing surgical interventions, the anesthesiologist should be informed about taking the drug (the possibility of developing acute respiratory distress syndrome in adults immediately after surgery).
In the case of simultaneous use of amiodarone and simvastatin, the dose of simvastatin should not exceed 10 mg per day due to the potential risk of developing rhabdomyolysis in such patients. In the case of simultaneous use of amiodarone and lovastatin, the dose of the latter should not exceed 40 mg per day. The patient should also be informed of the need to immediately consult a doctor in case of any unexpected muscle pain, muscle weakness.
Pregnancy and lactation
Use during pregnancy and lactation is possible only with life-threatening arrhythmias with the ineffectiveness of other antiarrhythmic therapy (causes fetal thyroid dysfunction). The safety and efficacy of use in children has not been determined.
Features of the effect of the drug on the ability to drive a vehicle or potentially dangerous mechanisms
During the period of treatment, it is necessary to refrain from driving and engaging in potentially hazardous activities that require an increased concentration of attention and speed of psychomotor reactions.
Overdose
Symptoms: bradycardia, atrioventricular blockade, lowering blood pressure, paroxysmal tachycardia of the "pirouette" type, aggravation of existing CHF, impaired liver function, cardiac arrest.